[Day 5] Prison Break 101: How to break a cell
We get to the last part of the the whole project,
where we get our product.
FINALLY!!! What a long wait.
Isolation
Our product here is Green Fluorescent Protein which
is an intracellular product. Therefore, in order to release
the protein within the cell, cell disruption needs to be carried out.
Three methods are performed in the experiment to lyse the bacteria cells.
 10mL of the culture broth was collected into a tube
for the whole experiment. Centrifuged the cells
at 10,000 rpm for 5 minutes which separates the cells
from the liquid broth and forms a pellet at the bottom
of the tube due to it being denser.
Since the liquid broth is less dense,
it constitutes the supernatant.
The supernatant is then transfered into a fresh new tube.
Both tubes were observed under the ultraviolet (UV) light
for product to confirm the results obtained.  Method 1: Use of Enzymes
Resuspend the pellet in 500µl of TE buffer of pH 7.5
with the use of a micropipette.
Ensure there are no visible clumps.
Add to drops of lysozyme into the resuspended cell pellet.
The lysozyme breaks down the cell wall, and hence,
releases the proteins within.
Allow proteins to act for 15 minutes.  Method 2: Freezing and Thawing
Place the tube in liquid nitrogen till
the contents are frozen. Thaw the tube in warm water.
Repeat the cycle of freezing and thawing twice more
to ensure complete disruption of the bacteria cell wall.
The cycle of freezing and thawing adds mechanical stress
to the cell wall, as the cell water content expands when frozen
and contracts when thawed.  Method 3: Sonication
This process is whereby ultrasonic waves are utilized
to cause cell disruption under the vibration pressure.
(Protective ear muffs must be worn when performing experiment)

Carry out sonication by puting it on ice
for 4 cycles of 25 seconds
with 10 seconds rest in between sonication cycles.
Centrifuge the tube for 20 minutes at 10 000 rpm.
Separate the supernatant and pellet.
Resuspend the pellet using 400µL of TE buffer.
Observe the tube under UV light to confirm the product.
Stage 2: Purification
Gel Filtration Permeation also known as
Size Exclusion Chromatography
will be performed to purify the extraction.
This method uses a column of polymer gel resins (Sephadex G75).
Due to the resins containing small pores,
small molecules will be able to diffuse through.
  As a result, as the extract is poured into the column, As a result, as the extract is poured into the column,
the bigger molecules will flow through faster,
as the smaller molecules spends more time diffusing
into the pores of the gel resins.
This allows the different molecules to be separated by size.
9 tubes are prepared and labeled.
2ml of ammonium bicarbonate is added into
the tube labeled “blank”. The buffer in the tube
is allowed to drain until it reaches just above
the gel bed, where the supernatant from the
isolation part is added in. Fractions of 2ml
of the drain are collected in the 8 tubes.
Ammonium bicarbonate is added
constantly to prevent the gel from drying.
The fractions collected in the 8 tubes are
subjected to Spectrophotometry to get the
absorbance readings at 476nm
(wavelength where GFP absorbs well).
  We use ammonium bicarbonate as the blank We use ammonium bicarbonate as the blank
to standardize and compare the absorbance values
with other fractions. We use gel filtration chromatography
to fractionate based on size the proteins in
a cellular extract. Its principle is that the
bigger molecules will flow through the column
faster without diffusing into the pores while
the smaller molecules get diffuse and interact
with the pores of the gel resins. Note that the column
should not allow being run dry; little cracks and
channels are formed when the column runs dry,
and separation of proteins is greatly compromised
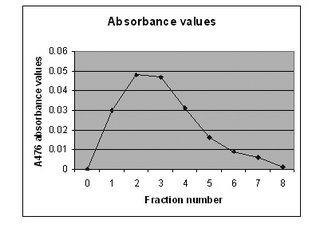
as a result. From the graph above, it clearly shows
that fraction number 2 and 3 have the most abs values.
This is because the big florescent molecules elude
quickly into both fractions and because
the size of the molecules is big, hence they
emit more florescent light. As for fraction number 1,
we believe that it did not show one of the most abs
values as the GFP has just started flowing down
and some of the molecules require some time to
flow the column as there are beads within
that are obstructing their flow. Another reason could be
other protein molecules with a size bigger than
the pore size of the beads are competing to flow
through the column with the GFP molecules.
From fraction 4 onwards, the abs values decrease
as the smaller molecules, like other proteins,
diffuse out from the beads and eluded. Gradually,
the number of fluorescent molecules decreases
with each fraction collected as most of them are
already collected at fraction 2 and 3. Therefore
only few of those are left to emit light. At fraction 8,
the abs value is almost zero, and we therefore
conclude that all the GFP had been eluted and
collected in the 8 tubes. From the results, we can
say that we have quite a pure preparation of GFP
as the protein of interest is well separated with
rest of the unwanted proteins.
-------------------------------------------------------------------
We have spoken.
|

 Reporter/Spokesperson Reporter/Spokesperson
Ernest Lai
 Leader Leader
Kelvin Tang
 Blogger #1/Editor Blogger #1/Editor
Ben Wong
 Blogger #2 Blogger #2
Huda
 Blogger #3 Blogger #3
Marcus Lim
 Visual Capturer Visual Capturer
Yong Sheng
 Secretary Secretary
Siu Ling
 Investigator [Chief] Investigator [Chief]
Jeffery Koh
 Investigator Investigator
Zeya
 Investigator Investigator
Jin Hong
 Investigator Investigator
Sze Ho
 Investigator Investigator
Shafiq
 Kenneth Lim
Kenneth Lim
PhotoBucket
Blogger
Blogskins
Design

|







 As a result, as the extract is poured into the column,
As a result, as the extract is poured into the column,
 We use ammonium bicarbonate as the blank
We use ammonium bicarbonate as the blank












